








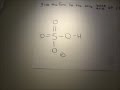











Write the formula of the conjugate base for acid NH4+? Answer Save. 2 Answers. Relevance. sakshi. 6 years ago. nh3 is the conjugate base for the acid nh4+.....as nh4+ will donate h+ it will act as an lowry bronsted acid.....after donating h+,it will form nh3, the conjugate base of ammonium.... 0 0. Viictoory. 6 years ago. NH4+ + H2O --> NH3 + H3O+ So NH3 is the conjugate base of NH4+ 1 0 ... Solved: What is the conjugate acid for NH2-? a. N3- b. NH2- c. NH4+ d. H+ By signing up, you'll get thousands of step-by-step solutions to your... Whats the formula for NH4 + SO32-+1. Answers (1) Gabriel Mitchell 17 January, 10:10. 0. I believe NH4 is ammonium SO3 2 - is sulfite is that what you were asking for. Comment; Complaint; Link; Know the Answer? Answer. Not Sure About the Answer? Find an answer to your question 👍 “Whats the formula for NH4 + SO32- ...” in 📗 Chemistry if the answers seem to be not correct or there’s ... Answer to Give the formula for the conjugate base of each acid. a. HNO2 b. NH4+ c. H202l... Problem: Give the conjugate base of the following Bronsted-Lowry acid: NH4+. FREE Expert Solution. 79% (93 ratings) Problem Details. Give the conjugate base of the following Bronsted-Lowry acid: NH 4 +. Learn this topic by watching Conjugate Acids and Bases Concept Videos. All Chemistry Practice Problems Conjugate Acids and Bases Practice Problems. Q. Label each of the following as being a ... Conjugate acid/base pairs means that one substance (the base) is the proton (H+) acceptor, and the other, the acid, is the proton donor. In an acid/base pair, the structures of the two compounds should be almost exactly the same, except the acid should have one more H than the base. Question: Give The Formula Of The Conjugate Base Of HSO4- . Give The Formula For The Conjugate Acid Of HSO4-. This problem has been solved! See the answer. Show transcribed image text. Videos. Step-by-step answer 04:16 0 0. Expert Answer 100% (54 ratings) Previous question Next question ... NH_3 NH_4^+ + H_2O -> NH_3 + H_3O^+ NH_4^+ is the acid because it donates an H^+ ion to the water. It then becomes ammonia (NH_3), which would be the conjugate base of NH_4^+. Give the formula of the conjugate base of each of the following. (Type your answer using the format CO2 for CO2, (NH4)2CO3 for (NH4)2CO3, [NH4]+ for NH4+, - 15415538 And the conjugate acid of bisphosphate ion, HPO_4^(2-), is H_2PO_4^-. As with all of these conjugate acid/conjugate base problems, I am simply simply exchanging a proton, H^+, and conserving mass AND charge. If mass and charge were not conserved, it would not be a realistic exercise. What are the conjugate bases of H_2SO_4, HSO_4^-, NH_3, HO^-, and NH_2^-? What are their conjugate acids?
[index] [1745] [9279] [2550] [1428] [2862] [6515] [1257] [6750] [2164] [897]
Calculating mass of the salt needed for a buffer solution. Here you will find curriculum-based, online educational resources for Chemistry for all grades. Subscribe and get access to thousands of top quality interact... Add some acid. Add some conjugate base. What's the pH? This is the old-school way. You can also use the Henderson-Hasselbalch Equation. In this example I use... Here I am just answering one of my hw problems. I will try to post as much hw as I can This is for an OCHEM CLASS. All the answers provided are checked by th... In the Brønsted-Lowry definition of acids and bases, a conjugate acid-base pair consists of two substances that differ only by the presence of a proton (H⁺).... 🚀To book a personalized 1-on-1 tutoring session:👉Janine The Tutorhttps://janinethetutor.com🚀More proven OneClass Services you might be interested in:👉One... Thanks to all of you who support me on Patreon. You da real mvps! $1 per month helps!! :) https://www.patreon.com/patrickjmt !! Example 2: https://www.you... http://www.chemistry.jamesmungall.co.ukAcid Base Chemistry7. Relative acidity and basicity -- competition for H+ a. pKa and pKb of conjugate acids and bases[... Finding the equation of a Polynomial from a graph by writing out the factors. This example has a double root. I show you how to find the factors and the lead... Use Bronsted Lowry Acid/Base Theory to identify conjugate acid base pairs.More free chemistry help at www.chemistnate.com
Copyright © 2024 m.bestslotplay.shop